- New


.....WHATSAPP ONLY ![]() 0808406035
0808406035
.....WHATSAPP ONLY ![]() 0808406035
0808406035
Treatment of flea and tick infestations in dogs.
This veterinary medicinal product provides immediate and persistent flea killing (Ctenocephalides felis and C. canis) and tick killing (Rhipicephalus sanguineus, Ixodes ricinus, I. hexagonus and Dermacentor reticulatus) activity for 1 month.
Fleas and ticks must attach to the host and start feeding to be exposed to the active substance.
Contraindications
Do not use in cases of hypersensitivity to the active substance or to one of the excipients.
Special warnings
Special precautions for safe use in the target species
Parasites must begin feeding on the host to be exposed to lotilaner; therefore the risk of transmission of parasite-borne diseases cannot be completely ruled out. The possibility that other animals in the same household may be a source of flea reinfection must be taken into account and these must be treated if necessary with an appropriate product. Fleas, at every stage of their development, can infest dog bedding and normal rest areas such as carpets and soft furnishings. In case of massive flea infestation, and at the beginning of control measures,these areas must be treated with a suitable environmental product and then cleaned regularly.
All efficacy and safety data were acquired from dogs and puppies 8 weeks or older and weighing 1.3 kg or greater. In the absence of available data, a veterinary surgeon should be consulted before treatment in puppies less than 8 weeks of age or with a body weight less than 1.3 kg.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Wash your hands after handling the product.
In case of accidental ingestion, contact a doctor immediately and show him the package leaflet or the label.
Pregnancy and breastfeeding
Laboratory studies on rats have not shown the existence of teratogenic effects. The safety of the veterinary medicinal product during pregnancy or breastfeeding has not been established.
Consult a veterinary surgeon before use during pregnancy and breastfeeding.
Fertility
Laboratory studies in rats have not shown the existence of adverse effects on the reproductive capacity of males and females. The safety of the medicine in breeding dogs has not been established.
Interaction with other veterinary medicinal products and other forms of interaction 

Overdose 

Dosage for each species, route(s) and method of administration 
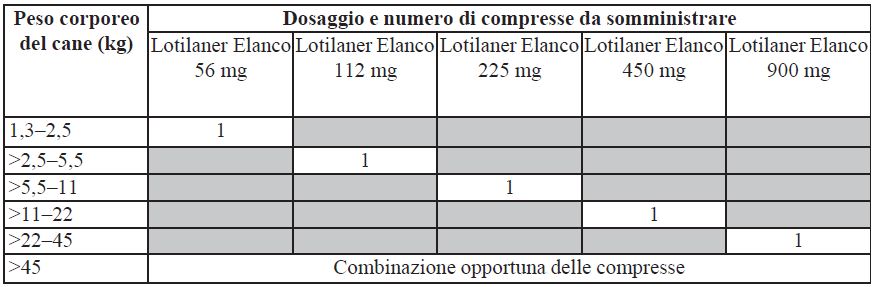
 The veterinary medicinal product should be administered according to the following table to ensure a lotilaner dose of 20 to 43 mg/kg body weight.
The veterinary medicinal product should be administered according to the following table to ensure a lotilaner dose of 20 to 43 mg/kg body weight. 

For dogs weighing more than 45 kg, use an appropriate combination of available doses to obtain the recommended dose of 20-43 mg/kg.
Under dosing may result in ineffective use and may encourage the development of resistance. To ensure correct dosage, determine body weight as accurately as possible.
Recommendations for correct administration
Lotilaner Elanco is a palatable flavored chewable tablet. Administer the chewable tablet with food or after meals.
For optimal control of flea and tick infestation, the product must be administered at monthly intervals throughout the flea and/or tick season, based on local epidemiological situations.
Special precautions for storage
Keep out of the sight and reach of children.
This veterinary medicinal product does not require any special storage conditions.
Do not use this veterinary medicinal product after the expiry date which is stated on the carton and blister after Exp. The expiry date refers to the last day of the month.
Special precautions for disposal
Medicines should not be disposed of via wastewater or household waste.
Use take-back systems for the disposal of unused veterinary medicinal products or waste resulting from the use of such medicinal products in accordance with local regulations and any national collection systems relevant for the veterinary medicinal product concerned.
Ask your veterinary doctor or pharmacist how to dispose of medicines you no longer need.
No customer reviews for the moment.