- New

.....WHATSAPP ONLY ![]() 0808406035
0808406035
.....WHATSAPP ONLY ![]() 0808406035
0808406035
Chewable tablets for the treatment of flea and tick infestations in cats
For the treatment of flea and tick infestations in cats.


For the treatment of flea and tick infestations in cats.
This veterinary medicinal product provides immediate and persistent flea (Ctenocephalides felis and C. canis) and tick (Ixodes ricinus) killing activity for 1 month.
Fleas and ticks must attach to the host and start feeding to be exposed to the active substance.
Special precautions for safe use in the target species
Parasites must start feeding on the host to be exposed to lotilaner; therefore the risk of transmission of parasite-borne diseases cannot be completely excluded.
The possibility that other animals in the same household may be a source of flea reinfection should be taken into account and these should be treated if necessary with an appropriate product. Fleas, at any stage of their development, can infest the cat's litter tray and normal resting areas such as carpets and soft furnishings. In the event of heavy flea infestation, and at the start of control measures, these areas should be treated with a suitable environmental product and then cleaned regularly. Acceptable levels of efficacy may not be achieved if the veterinary medicinal product is not administered with food or within 30 minutes of feeding.
Due to insufficient data to support efficacy against ticks in young cats,This product is not recommended for the treatment of ticks in kittens 5 months of age or younger. All safety and efficacy data have been acquired from cats and kittens 8 weeks of age or older and weighing 0.5 kg or more. In the absence of available data, a veterinarian should be consulted before treatment in kittens less than 8 weeks of age or with a body weight less than 0.5 kg.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Wash hands after handling the product.
In case of accidental ingestion, seek medical advice immediately and show the package leaflet or label to the doctor.
Pregnancy and breastfeeding
Laboratory studies in rats have not shown any teratogenic effects.
The safety of the veterinary medicinal product in cats during pregnancy and lactation has not been established. Consult a veterinary surgeon before use during pregnancy and breastfeeding.
Fertility
Laboratory studies in rats have not shown any adverse effects on the reproductive capacity of males and females.
The safety of the veterinary medicinal product in breeding cats has not been established. Consult a veterinarian before use in breeding cats.
Interaction with other veterinary medicinal products and other forms of interaction
None known. During clinical field studies, no interactions between lotilaner and commonly used veterinary medicinal products were observed.
Overdose
No adverse reactions were observed after oral administration to 8-week-old kittens, with a body weight of 0.5 kg, treated with overdoses exceeding 5 times the maximum recommended dose (130 mg lotilaner/kg body weight) on eight occasions at monthly intervals.
Adverse events
Target species: Cat.
Reporting of adverse events is important as it allows the continued monitoring of the safety of a product. If you notice any side effects, including those not mentioned in this leaflet, or if you think that the medicine has not worked, please inform your veterinary surgeon in the first instance. You can also report any adverse events to the Marketing Authorisation Holder using the contact details at the end of this leaflet or via the national reporting system: {national system data}
Posology for each species, route(s) and method of administration
For oral use.
The flavoured veterinary medicinal product should be administered according to the following table to ensure a single dose of lotilaner of 6 to 24 mg/kg body weight.
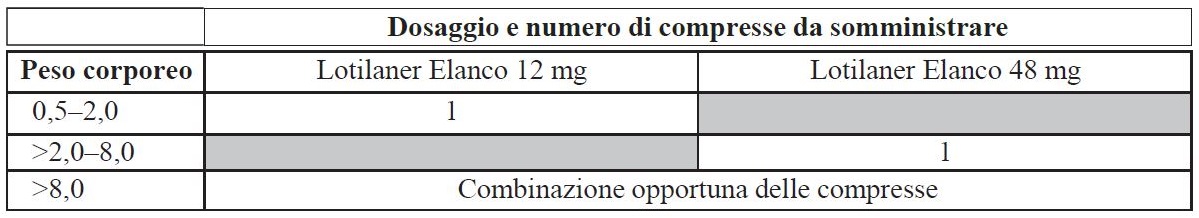
In cats with a body weight greater than 8 kg, use an appropriate combination of the available strengths to achieve the recommended dose of 6-24 mg/kg.
Under dosing may result in ineffective use and may promote the development of resistance. To ensure correct dosing, determine body weight as accurately as possible.
Recommendations for correct administration
Administer the veterinary medicinal product with food or within 30 minutes of feeding.
For optimal control of tick and flea infestations, the veterinary medicinal product should be administered at monthly intervals and continued during the flea and/or tick season according to local epidemiological situations.
Special precautions for storage
Keep out of the sight and reach of children.
This veterinary medicinal product does not require any special storage conditions.
Do not use this veterinary medicinal product after the expiry date which is stated on the carton and blister after Exp. The expiry date refers to the last day of that month.
Special precautions for disposal
Medicinal products should not be disposed of via wastewater or household waste.
Use take-back systems for the disposal of unused veterinary medicinal products or waste products derived from the use of such medicinal products in accordance with local requirements and any national collection systems relevant to the veterinary medicinal product concerned.These measures are to protect the environment.
Ask your veterinarian or pharmacist how to dispose of medicines you no longer need.
No customer reviews for the moment.